|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Non-heme Fe(II) complexes with tripodal nitrogen ligands, and reductive activation of dioxygen Dominique Mandon Laboratoire de Chimie Biomimétique des Métaux de Transition, Institut de Chimie de Strasbourg, UMR 7177 CNRS-UdS, bâtiment Le bel, 4 Rue Blaise Pascal – CS 90032 - F-67081 Strasbourg CedexFrance. mandon@unistra.fr
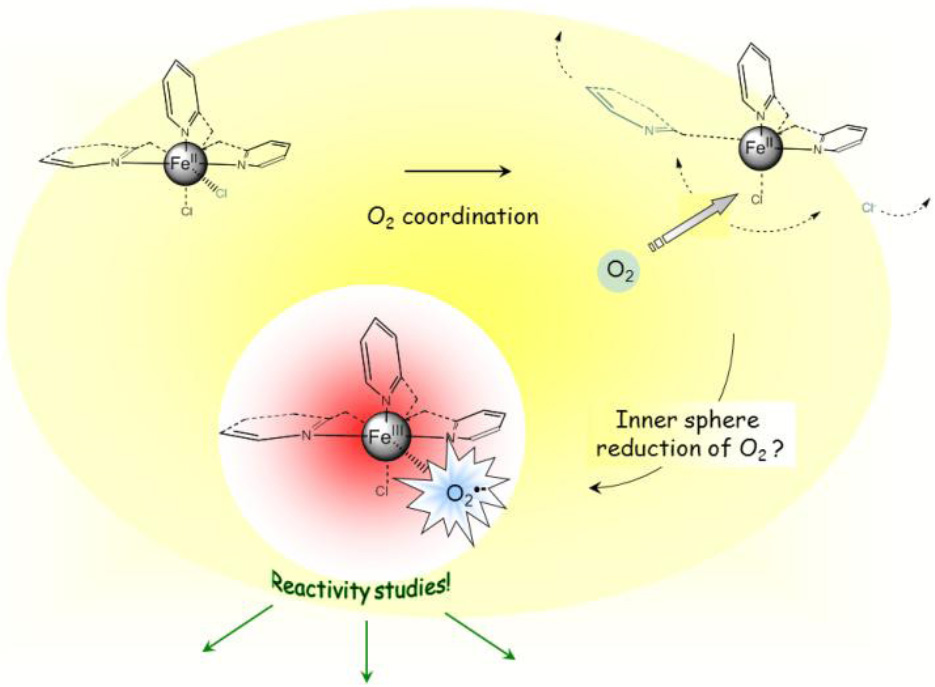
The inorganic chemistry of i ron complexes with tris(2-pyridylmethyl)amine-type ligands (TPAs) which developed over the last fifteen years has provided many examples of structural (access to high-valent species) or functional (biomimetic catalysis) synthetic analogues of active sites of non-heme iron proteins involved as enzymes in oxidation reactions. In most of the cases however have synthetic chemists used peroxides as oxygen donors, whereas Nature uses molecular oxygen: the number of reported examples of simple mononuclear complexes with such ligands that are able to react with molecular oxygen is limited, the metal being in that case generally activated by a non-innocent exogenous ligand (thiolate, catecholate or a -ceto carboxylate). FeCl2 complexes with TPA-type ligands display moderately positive Fe II/Fe III redox couples: they are a priori not supposed to reduce molecular oxygen. But FeCl 2 complexes with TPA-type tripods are definitely oxygen-sensitive, and undergo clean conversions, most of the time into µ-oxo diferric species. Additionally and depending on its structure, modification of the ligand may be observed. It seems that accessibility of O2 to the metal centre, as well as Lewis acidity of the iron represent crucial parameters with respect to the oxygen sensitivity. We shall present indirect evidence for coordination of O2 to the iron centre, leading to the reductive activation of molecular oxygen. We shall also discuss the characterization by a broad array of spectroscopic techniques of a transient species obtained upon oxygenation of an Fe II complex, which may be described as an [Fe +III] [O2 . -] species.
Selected references: Costas, M.; Mehn, M. P.; Jensen, M. P.; Que, L. Chem. Rev . 2004, 104, 939-986. Solomon, E. I.; Brunold, T. C.; Davis, M. I.; Kemsley, J. N.; Lee, S. K.; Lehnert, N.; Neese, F.; Skulan, A. J.; Yang, Y. S.; Zhou, J. Chemical Reviews2000, 100, 235-349. Abu-Omar, M. M.; Loaiza, A.; Hontzeas, N. Chemical Reviews2005, 105, 2227-2252. Special issue on dioxygen activation by metalloenzymes and models, Acc. Chem Res., 2007, 40, 465 – 634. Special issue: Forum on Dioxygen Activation and Reduction, Inorg. Chem.,2010, 49, 3555 – 3675, Mandon, D., Jaafar, H., Thibon, A., New J. Chem., 2011, 35, 1986 – 2000.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||