|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ferrocenyl anthracenes and triptycenes: From steric crowding to a molecular brake Michael J. McGlinchey University College Dublin, Ireland
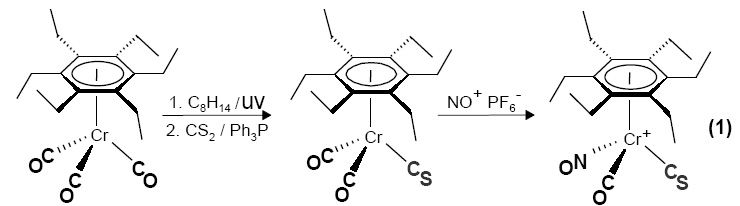
Our interest in sterically crowded molecules began in 1982 during a collaboration with Didier Astruc on the structure and dynamics of [(C6Et6)Fe(C5H5)]+.1 This was extended to (C6Et6)Cr(CO)3 whereby the question arose of the possibility of correlated rotation of the ethyl groups and the tripod; this problem was resolved via the chiral cation [((C6Et6)Cr(CO)(CS)(NO)]+(1). 2 Other sterically hindered systems that were studied included (C5Ph5)Fe(CO)(CHO)PMe3 , [C7Ph7]+, hexanaphthylbenzene and ferrocenyl-pentanaphthylbenzene.
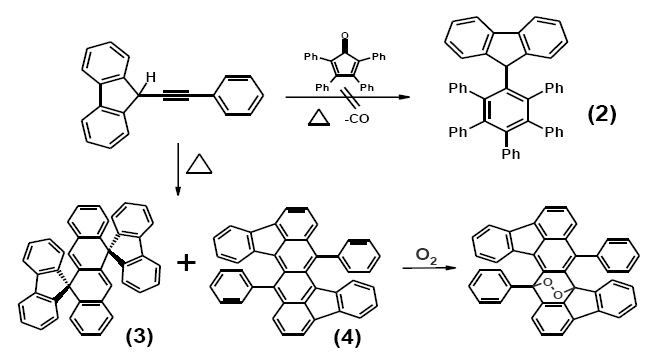
An attempt to prepare a molecular brake by controlling an η6 to η5 haptotropic migration of a CpFe fragment across the fluorenyl surface of 9-fluorenylpentaphenylbenzene (2) was frustrated by the failure to prepare the ligand, whose attempted synthesis via the Diels-Alder reaction of tetraphenylcyclopentadienone and 9-phenylethynylfluorene led instead to electroluminescent tetracenes (3) and (4).3 Moreover, elucidation of the mechanism of tetracene formation revealed the intermediacy of a series of C 2-symmetric bis(fluorenylidene)cyclobutanes.4
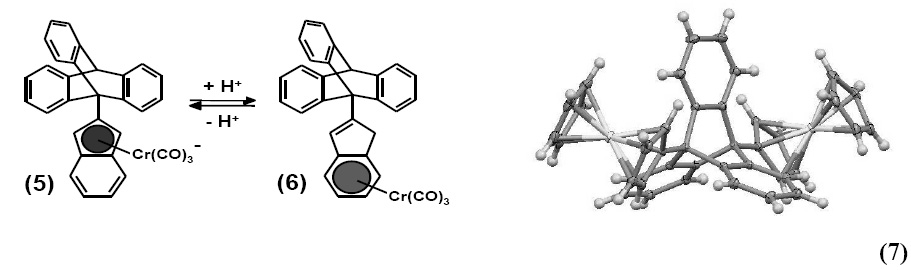
Demonstration of the first organometallic molecular brake was finally achieved by the base-activated migration of a Cr(CO)3 moiety across the indenyl surface of 9-(2-indenyl)triptycene, as in (5) and (6). 5 With the goal of controlling such a haptrotropic shift process electrochemically, rather than by changing pH, the structures and dynamics of mono- and bis-ferrocenyl-anthracenes and ‑triptycenes, e.g. (7), were investigated and yielded a set of organometallic molecular dials.6
1. -JACS 1982, 104, 7549. 2.- JACS 1991, 113, 1177. 3.- Org. Lett. 2004, 6, 787. 4.- Chem. Eur. J. 2006, 12, 3275. 5.- Chem. Eur. J. 2009, 15, 1836. 6.- JACS 2010, 132, 17617.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||