|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Investigating the electronic structure and reactivity of f-element compounds.
Jennifer C. Green Department of Chemistry Oxford University Oxford, UK
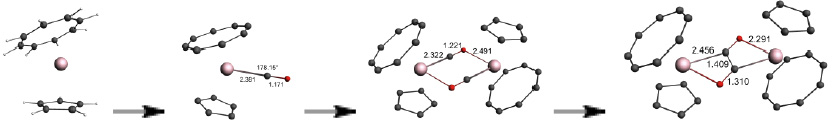
The mechanism of reductive oligomerization of CO by some U(III) sandwich compounds has been investigated by computational studies. It is proposed that the initial C-C bond formation proceeds stepwise via formation of a U(III)CO complex which dimerizes through coordination of O to the opposing U, followed by C-C coupling of the adjacent CO carbons. The degree of reduction of the CO units is sensitive to the computational methodology.
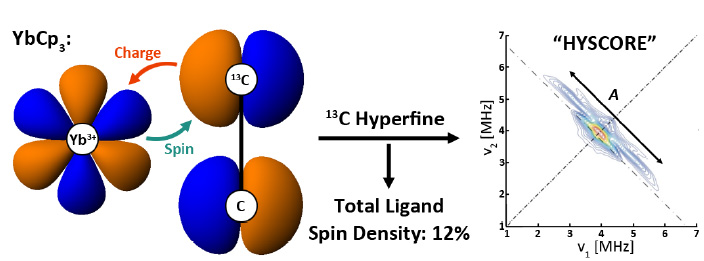
Evidence is presented that the electronic ground state of tris-cyclopentadienyl ytterbium (YbCp 3) exhibits significant covalency in the ytterbium 4f shell that can be represented by the superposition of an ionic configuration Yb(III):4f 13(Cp 3) and a charge-transfer configuration Yb(II):4f 14(Cp 3) -1. The relative weights of these configurations were determined by using (i) the difference in their 4f photoionization cross-sections, (ii) the accumulation of spin-density centered on the 13C atoms of the Cp ring, as measured by a pulsed EPR (HYSCORE) experiment, (iii) the reduction in the spin-density in the 4f-shell, manifest in the 171Yb hyperfine interaction, and (iv) the principal values of the g-tensor, obtained from the EPR spectrum of a frozen glass solution at 5.4 oK. Each of these methods finds a density attributable to the charge transfer configuration in the range 12-15%. This configuration interaction (CI) also accounts for the highly anomalous energies, intensities and vibronic structure in the “f-f” region of the optical spectrum, as well as the strict adherence of the magnetic susceptibility to the Curie Law in the range 30 – 300 oK.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||