|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Engineering of neutral cyclometallated platinum(II) complexes incorporating acetylide ligands for chemosensing Jean-Luc Fillaut Sciences Chimiques de Rennes UMR 6226 CNRS-Université de Rennes 1.
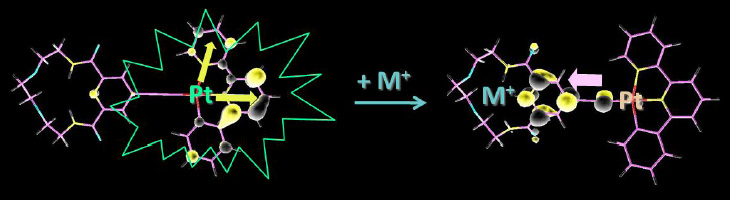
Significant work in the field of responsive materials has recently focused on cyclometalated d8 square-planar Pt(II) complexes owing to their photophysical properties. Among them, (C^N^N)PtX complexes (C^N^N = 6-phenyl-2,2'-bipyridine (phbpy), X = halide, acetylide, etc.) are particularly interesting because of their intense phosphorescent emission. The ability to vary the ancillary ligand X is an elegant strategy to induce structural modification of the [(C^N^N)PtX] complexes and to tune their photophysical properties. In these series, acetylide derivatives are of special interest, not only because the strong-field nature of the acetylide ligand can help to augment the emission quantum yield, but also due to the facility with which functionalized terminal aryl-alkynes can be accessed, opening a way to new systems containing a diversity of host receptors. On the other hand, heavy metal ions play a vital role in various domains such as analytical chemistry and biological and environment sciences. It is well known that Zn2+, Fe3+, and Cu2+ are the three most abundant essential heavy metal ions in the human body and participate in diverse biological processes. Pb 2+ and Cd 2+ are also considered as significant pollutants, which can cause serious environmental and health problems. Therefore, the design and development of potential chemosensors for the detection of heavy metal ions are very important. Aiming at exploring the responses toward various heavy metal ions, we designed novel platinum(II) complexes with functionalized acetylide ligand as specific receptors for heavy metal ions, where the cyclometalated [Pt(tBu2-C^N^N)(C≡C-R)] (tBu2-C^N^N = 4,4’-di(tert-butyl)-6-phenyl-2,2’-bipyridine) chromophore served as a signal emitter. Absorption and luminescence sensing behavior of these platinum(II) complexes can be modulated by variations in the binding sites of the acetylide ligands. Upon binding to specific metal ions both the absorption and emission properties of these platinum(II) complexes can be strongly affected so that they can be helpful to be used as models of highly sensitive chemosensors.
We will describe joint TD-DFT and experimental studies of a series of [Pt(tBu2-C^N^N)(C≡C-R)] complexes, where R groups are receptors for heavy metal cations (Scheme). These studies highlight the predominant role of the energy levels of the LUMOs in these complexes as the key to tune their emission, giving rise to unprecedented switches of luminescence. The design concept employed suggests that novel future strategies for sensing of important metal ions based on perturbations in the phosphorescence of 3rd row transition metal complexes could realistically be established.
References: New J. Chem. 2011 , 35, (cuvée bordelaise spéciale !). Coord. Chem. Rev. 2011 , 255, 2448-2457. Eur. J. Inorg. Chem . 2011 , 1255–1259 ; Dalton Trans. 2010, 39, 707–710; Chem. Commun.2008, 4333-4335.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||