|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
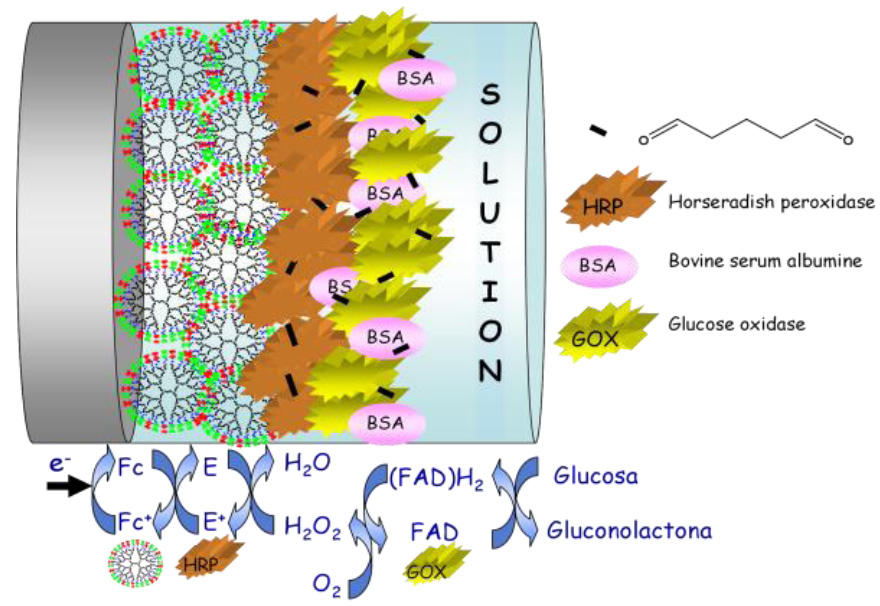
"Redox-active Organometallic Dendrimers: Electrochemical Biosensors” Carmen M. Casado, a Beatriz Alonso, a José Losada, b M. Pilar García-Armada a Dpto. Química Inorgánica, Universidad Autónoma de Madrid, Madrid, Spain b Dpto. Ingeniería Química Industrial Universidad Politécnica de Madrid, Madrid, Spain Poly(ferrocenyl)-based macromolecules have demonstrated to be excellent candidates to play a key role in understanding biological electron transfer and for application in the chemical modification of electrodes, as electrode mediators, electrochemical sensors as well as in other fields. Since 1994, the tailored design and synthesis of dendritic macromolecules containing multiple organometallic units with specific applications have become a high-priority objective in our laboratory. We have reported the synthesis of several families of silicon- and nitrogen-based organometallic dendritic molecules decorated, in most cases, with either ferrocene or cobaltocenium redox-active moieties as surface functional groups. Electrochemical properties of both metallocenes are dominated by a reversible monoelectronic oxidation (ferrocene) or reduction (cobaltocenium), which can be easily obtained at very accessible potentials. Some of these dendritic molecules have been successfully used in the modification of electrode surfaces, as electron transfer mediators in amperometric biosensors and as exo-receptors for sensing anions.1
In this talk, we will mainly focus on recent work in our group on the development of new electrochemical bioelectrocatalysts and sensors for the molecular recognition of biomolecules. . 1"Redox-Active Organometallic Dendrimers as Electrochemical Sensors" in Designing Dendrimers, S. Campagna, P. Ceroni, and F. Puntoriero (ed.), John Wiley & Sons, Hoboken, NJ, C. M. Casado, B. Alonso, J. Losada and M. P. García-Armada, ISBN-10: 0-470-43355. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||