|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FERROCENE: STILL GOING STRONG
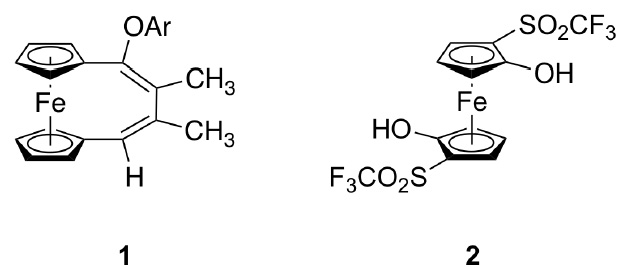
Holger Butenschön Institut für Organische Chemie, Leibniz Universität Hannover Schneiderberg 1B, D-30167 Hannover, Germany holger.butenschoen@mbox.oci.uni-hannover.de In the context of our interest in ferrocene based molecular wires 1,1'-dialkynylferrocenes were investigated with respect to the possibility to replace some, not all of the 1,4-phenylene units in oligophenyleneethynylenes (OPEs) by ferrocene units. The idea was to obtain molecular wires with a limited conformational flexibility (foldable ruler) and a three dimensional design. [1] In addition to palladium catalyzed coupling reaction alkyne metathesis was considered an alternative coupling procedure. Here, in addition to some reactions along these lines, an unexpected interannular coupling between 1,1'-dialkynylferrocenes and phenols will be presented resulting in [4]ferrocenophane derivatives such as 1. [2]
In the second part our attempts to generate ferrocyne (1,2-dehydroferrocene) and related species will be summarized. Here, triflate elimination was considered the method of choice. Instead, an unexpected anionic thia Fries rearrangement gave rise to the formation to 2-(trifluoromethylsulfonyl)ferrocenols. More strikingly, this reaction occurs twice, when ferrocenyl-1,1'-ditriflate was submitted to the reaction conditions. While due to the planar chirality of the system two diastereomeric ferrocene-1,1'-diols had to be expected, only 2 is exclusively formed in high yield as a rare example of interannular stereoselection in a ferrocene system. [3] Finally a different attempt to get access to ferrocyne will be presented on a preliminary basis. [4]
[1] I. Baumgardt, H. Butenschön, Eur. J. Org. Chem. 2010, 1076-1087. [2] J. Ma, B. Kühn, T. Hackl, H. Butenschön, Chem. Eur. J. 2010, 16, 1859-1870. [3] G. Werner, C. W. Lehmann, H. Butenschön, Adv. Synth. Catal. 2010, 352, 1345-1355. [4] G. Werner, Dissertation, Leibniz Universität Hannover, 2011.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||